In 10 seconds? Scientists have discovered a way to process wastewater that produces hydrogen. The tech one day could be used for energy storage or powering zero-emission cars.
Why is it a big deal? We save money and energy on treating wastewater (which is normally a very energy-demanding process) and we get hydrogen fuel for our future, climate-friendly cars! Consider this: transportation is responsible for a huge, chunk over 20% of our global greenhouse gas ‘emission pie’. And road transport accounts for about 75% of that 20%. This means that if we managed to turn all those trucks, cars, and buses into zero-emission vehicles, we'd take a big step toward reducing greenhouse gas emissions. Hydrogen is currently touted as a possible energy source for such vehicles.
Sounds like great news but how is hydrogen produced from wastewater? Researchers used microbial fuel cells (MFC) based on the process of microbial decomposition of organic material. The process involves attaching electron-transferring microorganisms, or exoelectrogens to an anode (i.e. the negative terminal in a battery) within a reactor material. Electrons are then diverted to an oxygenated cathode (the positive terminal of the battery). This process has been used to treat wastewater since 1991. However, it produces that global warming inducing gas, carbon dioxide (CO2), and electricity. So how can be this good to cut emissions? Here is the novelty element: by adding an external electrical current to the MFCs, and creating a microbial electrolysis cell (MEC), hydrogen can be produced during the treatment of wastewater.
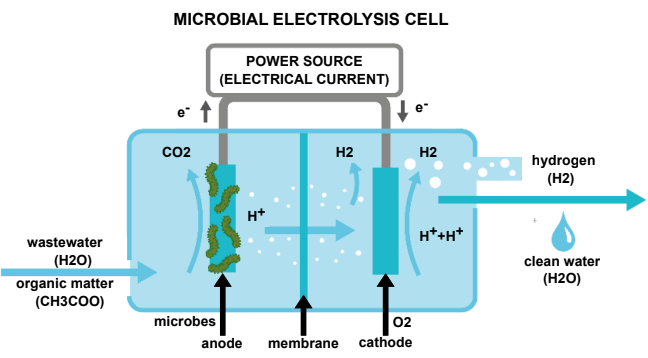
Awesome, so we get hydrogen but why do need to produce it from wastewater? As we move from traditional fuels, such as petrol and diesel, to cut greenhouse gas emissions in line with the Paris Agreement, there is a greater need to source more sustainable fuel for zero-emission cars. Hydrogen production from conventional sources (such as oil, gas, and coal) releases up to 29.33 kg of CO2 per kg of hydrogen due to the amount of energy needed and processes involved. By using MEC technology to produce hydrogen, CO2 emissions can be reduced to just 0.87 kg CO2 per kg of hydrogen. The numbers speak for themselves, right?
What are the other benefits of using MEC technology? Wastewater treatment uses 1-4% of our electricity consumption, which also adds significantly to our greenhouse gas emissions. By switching to MEC technology to treat wastewater, we can also cut emissions associated with energy generation and there could be up to a 42% reduction inemissions compared to existing wastewater treatment systems. Using MECs to produce hydrogen may also result in producing energy, further increasing sustainability.
Are there any negatives? Currently, the technology isn’t very affordable, at $3,017 per ton of hydrogen, compared to conventional sources ($603 - $1,810 per ton of hydrogen). This is mostly due to the cost of materials needed to make the reactor. However, scientists from the University of Warwick in the UK have recently created a recycled carbon fiber fabric that can be used to create the anode. This new material is not only more sustainable as its recycled, but also saves money at $7.22 per m2, compared to $115.44 per m2 for the currently used graphite fabric; a total cost saving of 93%. Furthermore, the recycled carbon fiber fabric produces more hydrogen than the graphite fabric, which is another win!
How much wastewater? A lot!
Did you know that the US has over 17,000 wastewater treatment plants and 1.300,000 miles of sewers?
They collect over 34 billion gallons of wastewater generated daily in the country.
According to the EPA, the average American household goes through more than 300 gallons per day.
So, imagine the energy and carbon savings that can be achieved in the treatment of this massive amount of water and generating fuel for cars that don’t contaminate the air!
Chloe has curated 10 research papers, saving you 35 hours of reading time.







