In 10 seconds? Immune checkpoint inhibitors are recently developed cancer immunotherapies that have helped treat advanced-stage cancers–such as skin and lung cancer.
What’s the story? In our last Immunity vs Cancer digest, we learned how cancer cells can evade being killed by immune cells called T cells by ‘coating’ themselves with molecules called checkpoints, which work to inactivate T cells. The discoveries of immune checkpoints have led to the development of a new class of cancer drugs called ‘immune checkpoint inhibitors (ICI)’, or checkpoint blockade therapies.
Wait! Why do checkpoint molecules exist if they can promote cancer? As important as it is for our immune system to build an army to respond to invaders, it’s equally important for the immune army to disband after it performs its duties. Why? As it fights, our immune cells multiply and spit out loads of toxic substances that can cause our bodies more harm than good if left unchecked. Hence the evolution of checkpoint molecules to help our bodies maintain a balance between wartime and peace.
So, immune checkpoints are natural? Yes! Usually, certain immune cells (called macrophages) coat themselves with checkpoint molecules and are responsible for dampening T cells' killing activity. However, through mutations or a host of other sneaky tactics, cancerous cells can manipulate the checkpoint process and avoid being killed by T cells.
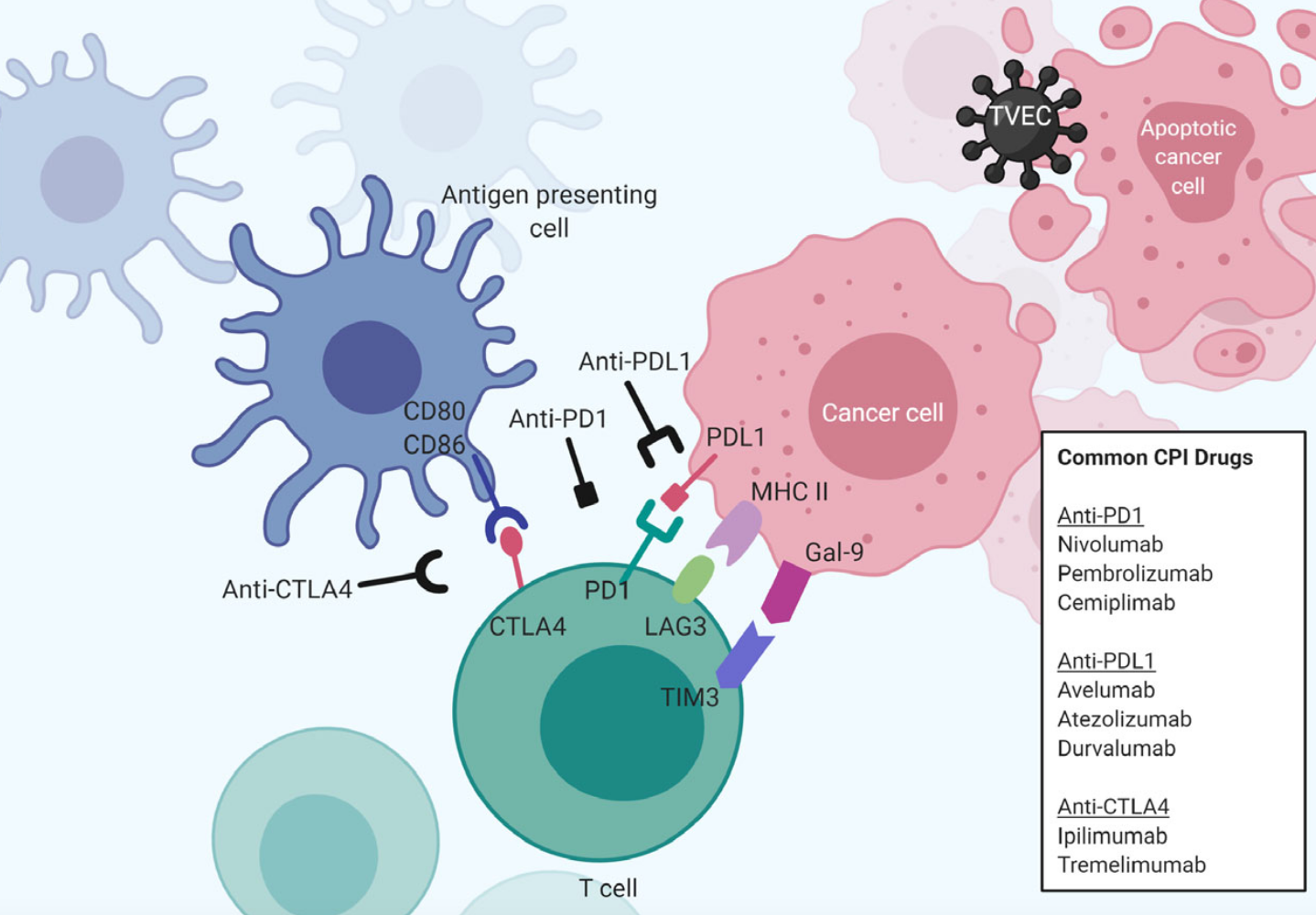
Hold up–how does this go down? Immune checkpoints use a receptor-coreceptor strategy–a method that cells use to communicate with each other. Here is how it works: cells coat themselves with compatible proteins (AKA receptors) that function similarly to a lock and key. When a cancer cell coats itself with checkpoint molecules that are compatible with their partner molecules on T cells, they can lock together and set off a chain of reactions inside T cells that make them stand down–stopping them from attacking cancer cells. And this, subsequently helps cancers grow.
Right. So tell me about how this relates to cancer treatments. On it! Scientists have developed a class of cancer immunotherapies called ‘immune checkpoint inhibitors (ICI)’ that prevent checkpoint molecules from stopping killer T cells from attacking cancer.
How do they work? Remember antibodies? The things people were hoping their immune system generated in response to COVID-19 vaccinations to be protected from infection? Well–ICI are antibodies! But instead of being generated by our immune systems to block viral infection, they are generated in a laboratory to specifically block the interaction between checkpoint molecules and their co-receptors (e.g. PDL-1/PD-1 or CD80/CTLA4).
Enough of the mechanics–how do these help patients? Right away! Checkpoint blockade therapies have made the difference between life and death for some patients with advanced-stage cancers (the toughest ones to treat!). First, you must meet PD-1 (Programmed Death 1)–one of the first checkpoint molecules identified. Treatment with an anti-PD-1 checkpoint blockade therapy can successfully reduce tumor sizes (and sometimes make them disappear) in advanced melanoma patients (31-44%), non-small-cell lung cancer patients (19-20%), and renal cell carcinoma (22-25%) patients. The first checkpoint blockade therapies were approved by the US FDA for patient care in 2015.
So, there are others? Beyond PD-1, there is a slew of other checkpoint molecules (e.g. CTLA4, LAG-3, TIM-3), and scientists continue to hunt for more. Doctors have been testing different checkpoint blockade therapies (AKA different antibodies specific for different checkpoint molecules) in many types of cancer–at different stages and in combination with other cancer treatments. All these clinical studies are leading to frequent new FDA approvals–meaning more treatment options for cancer patients!
That’s amazing! But what’s the catch? How’d you know? As I mentioned–immune checkpoint molecules evolved to prevent our immune system from going crazy–and checkpoint blockade inhibitors unleash all T cells whether they are specific for cancer or not. That means these drugs can lead to a host of autoimmune problems (AKA our own immune system causing us to be sick). The most common autoimmune side effects are gastrointestinal problems (Think: diarrhea) and skin reactions (Think: rashes) but pretty much every part of a patient's body–from our liver to the brain– can be impacted by autoimmunity, and some of these reactions can be life-threatening. Of course, scientists and doctors are working on ways to predict successes with checkpoint blockade inhibitors and minimize side effects. Stay tuned for our next Immunity vs Cancer digest to learn about the progress they’ve made so far.
How to tell if a drug is an antibody:
Checkpoint blockade inhibitors are far from the only monoclonal antibodies (Think: clones of one type of antibody made in a lab) used to treat medical conditions.
Want to be able to tell if a drug is an antibody? There’s a simple trick. Look at the ending of its non-proprietary name (not the commercial name). If it ends in
- -ab = monoclonal antibody or mAb
- -momab = mAb made in a mouse
- -mumab = mAb made in a human
- -zumab or ximab = mAb from a mouse modified to be more human-like
Dr. Talia Henkle has distilled 4 research papers, saving your 14 hours of reading time
The Science Integrity Check of this 3-min Science Digest was performed by Flávia Oliveira Geraldes.